Bhiwandi, Thane, Maharashtra
- GST NO. : 27AKFPM8972N1Z9
View Mobile Number
| Business Type | Manufacturer, Exporter, Supplier, Retailer |
| Grade | IP / BP / EP / USP |
We have well-connected and huge warehouse that helps us in keeping large stock under suitable conditions. Also, we keep it updated to meet set industry standards. With our inventory management system, we are capable to undertake urgent and massive requirements of the buyers at a time.
Details
-
Article - 1206-99
-
Batch No. - MPS/008/10/16
-
Mfg. Date - 10/2016
-
Exp. Date - 09/2019
Remark : In the opinion of the undersigned the sample tested to above is of standard quantity of In House Specification and complies I.P. Specification with respect to the above mentioned test
Abstract From Factory Record
-
Analysed by - Q.C. Chemist
-
Approved by - Q . C. Manager
Disclaimer of liability : The information in this SDS was obtained from sources which we believe are reliable. However, the information is provided without any warranty, express or implied, regarding its correctness. The conditions or methods of handling, storage, use or disposal of the product are beyond our control and may be beyond our knowledge. For this and other reasons, we do not assume responsibility and expressly disclaim liability for loss, damage or expense arising out of or in anyway connected with the handling, storage, use or disposal of the product. Buyer should ascertain & verify the data and analyse on its own before using the product. This data I information is the indication and not final. Products covered by valid patents in a country are not offered & I or supplied there. The customer is responsible for verification of the patent position. Product are not offered in the countries where they are covered under Patent. However, ultimate responsibility remains of the customer.
Analytical Report
| Test | Specifications | Results |
| Description | A white crystalline powder or colorless crystals | White crystalline powder. |
| Solubility | Freely soluble in water, soluble in methanol, Sparingly soluble in alcohol; Slightly soluble in IPA | Complies |
| Identification | By IR: IR absorption spectrum of sample should be concordant with that of Metoprolol succinate WS | Complies |
| pH | Between 7.0 to 7.6 | 7.42 |
| Heavy Metals | NMT 10 ppm | Complies |
| Loss On Drying | NMT 0.2% w/w | 0.13% w/w |
| Sulphated Ash | NMT 0.1% w/w | 0.06% w/w |
| Assay by HPLC (ODB) | NLT 98% w/w & NMT 102% w/w | 0.99 |
| Related substances by HPLC | Metoprolol related compd A: NMT0.1% | 0.057% |
| Metoprolol related compd B: NMT0.1% | 0.005% | |
| Metoprolol related compd C: NMT0.1% | 0.009% | |
| Metoprolol related compd D: NMT0.1% | Not Detected | |
| Any maximum unknown impurities: NMT 0.1% | 0.020% | |
| Total Impurities: NMT 0.5% | 0.124% |
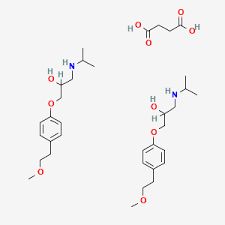
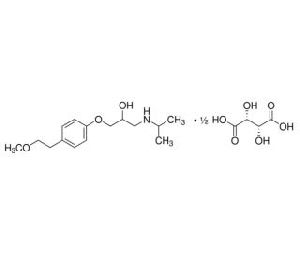
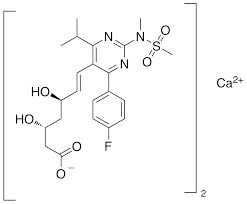
Looking for "Metoprolol Succinate" ?
Explore More Products